The European Commission will finalise a proposal by the end of the week to require pharmaceutical firms to register their vaccine exports from the European Union, while insisting it had no plans to impose an export ban.
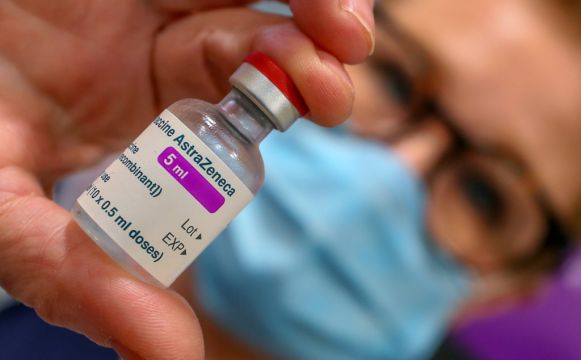
EU countries learnt late last week that deliveries of the Covid-19 vaccine from AstraZeneca, which is expected to be approved on Friday, would be some 60 per cent lower in the first quarter than initially indicated.
There have also been reduced deliveries of a vaccine jointly produced by Pfizer and BioNTech.
The European Commission, which has ordered hundreds of millions of doses of both vaccines, said on Tuesday it would have a proposal ready by the end of the week on vaccine exports, insisting it was merely to increase transparency.
"It's worth noting we are not planning to impose export bans or export restrictions," EU trade commissioner Valdis Dombrovskis told a news conference.
The bloc did require exports of protective equipment, including masks, to be authorised by individual EU countries at the start of the pandemic.
Contracts
The European Union can already monitor exports via customs data, but wants clearer data as it seeks to hold pharmaceutical companies to their contracts.
"This is based on the idea that the European Commission has spent quite a lot of funds on the development of these production capacities to make sure there are sufficient doses available for the European Union and beyond," a spokesman said.
Under EU contracts with Covid-19 vaccine makers, the EU has given up-front payments to secure doses in advance and boost firms' production capacity.
Pharmaceutical companies are usually required to deliver ordered doses under quarterly schedules.
Practical details of the registration scheme were still to be worked out, he said.
The EU executive is set to hold a second round of crisis talks with AstraZeneca on Wednesday.