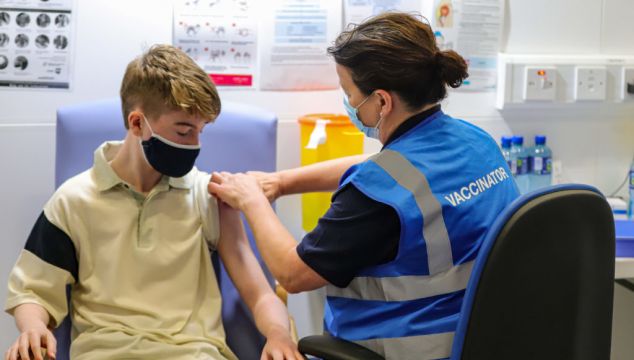
A Covid-19 vaccine booster dose is to be offered to children aged 12-15 in Ireland, the Minister for Health has confirmed.
The additional shot will be offered on the advice of the National Immunisation Advisory Committee (Niac), a recommendation which was endorsed by the country’s chief medical officer.
It comes as another 3,473 positive cases of Covid-19 were confirmed by PCR test on Monday. A further 2,865 people registered a positive antigen test through the HSE portal.
Over the weekend, 8,198 cases of Covid-19 were confirmed by PCR test and 5,650 people registered a positive antigen test through the HSE portal.
A booster dose of the mRNA vaccine Comirnaty, the vaccine manufactured by Pfizer/BioNTech, will now be given at an interval of six months or longer since completion of a child’s primary vaccine series.
For children who have experienced a breakthrough infection of Covid-19, the booster dose should be deferred for at least six months following the onset of infection.
“Niac have indicated that vaccine efficacy against symptomatic infection and hospitalisation was restored to 60-75 per cent and 90 per cent respectively, two to four weeks after administration of a booster in the adult population,” said Minister for Health Stephen Donnelly.
“Evidence from Israel’s booster programme shows a significant reduction in the confirmed rate of infection in children and young people aged 12-15 years following the booster dose, compared to those of the same age who were vaccinated with the primary series five to six months earlier.
"The benefits [of] vaccination – the prevention of serious illness and death – are clear, however, the reduction in [the] rate of infection is important as Ireland removes some of the last public health restrictions in place."
While primary vaccination with mRNA vaccines has been authorised by the European Medicines Agency (EMA) for this age group, no such authorisation exists in respect of a booster dose.
An application to authorise Comirnaty as a booster for adolescents from 12 years of age is currently being assessed by the EMA.
The Department of Health said that in the context of such off-label use, it and the HSE “will ensure that special attention is paid to the provision of support and guidance information as part of the informed consent process for children and young people and their parents/guardians.”
It comes after immunology expert Professor Kingston Mills said there is a case for giving a booster dose to children as an extra level of protection against a new sub-variant of Omicron.
-Additional reporting by PA