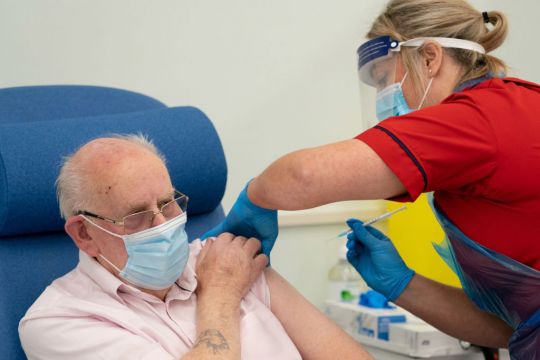
A redress scheme for those who suffer extreme reactions to the Covid-19 vaccine is still being developed, the Oireachtas Health Committee will hear today.
The opening statement to the committee from Prof Karina Butler, a consultant paediatrician and chair of the National Immunisation Advisory Committee, notes that such a scheme has been recommended by the World Health Organisation (WHO).
“A further challenge is to finalise the development of a national injury redress programme to provide support and care in the event that any vaccine recipient develops a serious vaccine reaction as recommended by the World Health Organization,” she said in the statement, seen by The Irish Examiner.
“Regarding safety, local site reactions and, less commonly, more general short‐term reactions – fatigue, flu-like symptoms, fever – can be anticipated.
“These events occurred at increased frequency following the second vaccine dose and were more common in the younger age groups.
“These effects are partly a result of the efficacy of the vaccines in stimulating the immune system.
“We will know more detail about the side‐effects when the licence documentation is published by the EMA [European Medicines Agency].
“Reports from the regulatory agencies in the US, UK and Canada raised no significant safety concerns.
“Report of anaphylactic reaction in two vaccines in the UK outside of the clinical trial naturally draw attention. Vaccine-associated anaphylaxis [severe immediate allergic reaction] is extremely rare, estimated at 1.31 per million vaccine doses. Its occurrence in the UK is being investigated.”
According to reports, the two reactions in the UK were suffered by individuals who have a history of allergic reactions and have both since recovered.
The State's plan to vaccinate the population was laid out in two separate documents – a high-level strategy and an implementation plan ‐ approved by the Government on Tuesday.
Prof Brian MacCraith, chair of the high-level task force on Covid‐19 vaccination, said in his statement to the committee that the programme to roll out the vaccine is “unparalleled”.