The Irish-born executive director of the European Medicines Agency (EMA), Emer Cooke, has described the efficacy of the Moderna and Pfizer Covid-19 vaccines in trials in children aged 12-15 as “impressive”.
The performance of the mRNA vaccines, which show efficacy levels of 90 per cent in older populations, has been replicated in children, she said on Thursday.
Both pharmaceutical companies had been asked to extend their trials “down through the age groups” so there would be additional information as it was required. A trial on children aged 12-15 was held on 4,000 subjects.
This was a “robust” database, she told RTÉ radio’s News at One. The EMA was also monitoring “real world” use of the vaccine in that age cohort in both the USA and Israel.
Even with the differences in metabolism speeds between children and adults, there was no evidence of a difference in efficacy of the vaccine between the age groups, she said.
“This gives us the confidence that the vaccine performs the same in children as in adults,” she said.
On the issue of the rare side effect of inflammation of the heart (myocarditis), Ms Cooke acknowledged that there had been a slightly greater incidence as trials moved down the age groups, but that such cases were still very rare, with a risk of one in 20,000.
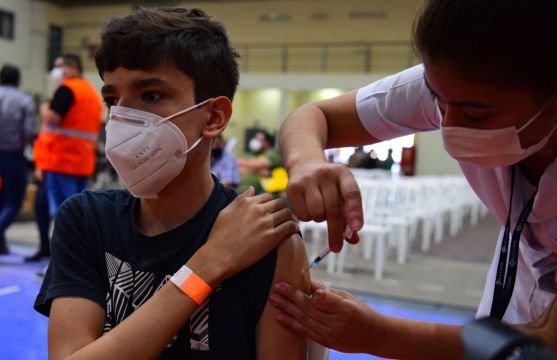
Approved for children
Minister for Health Stephen Donnelly confirmed earlier this week that the Covid-19 vaccination programme will be extended to all those aged 12-15 following a recommendation by the National Immunisation Advisory Committee (Niac).

Both mRNA vaccines – Pfizer and Moderna – have been approved by the EMA for use in this age group.
Meanwhile, those aged are 16 and 17 can currently register for a Covid-19 vaccine and can also attend walk-in vaccination clinics, which are operating across the country over the August bank holiday weekend.
Ms Cooke said studies were ongoing on the need to “tweak” vaccines to respond to new variants. The EMA had issued guidelines last April, she said, but so far there had not been a need, but if necessary the agency would be able to move quickly.
A fifth vaccine – Novovax, developed in the USA – was still being examined by the EMA, but there remained an issue on the company’s ability to manufacture to scale, she said. A decision could be reached in the third quarter, she said.