Journalist and TV presenter Joan Bakewell is threatening the UK government with legal action over delays to the second dose of the Pfizer/BioNTech vaccine.
The Labour life peer said there were grounds to show the decision taken by ministers to delay the second dose by up to 12 weeks was unlawful.
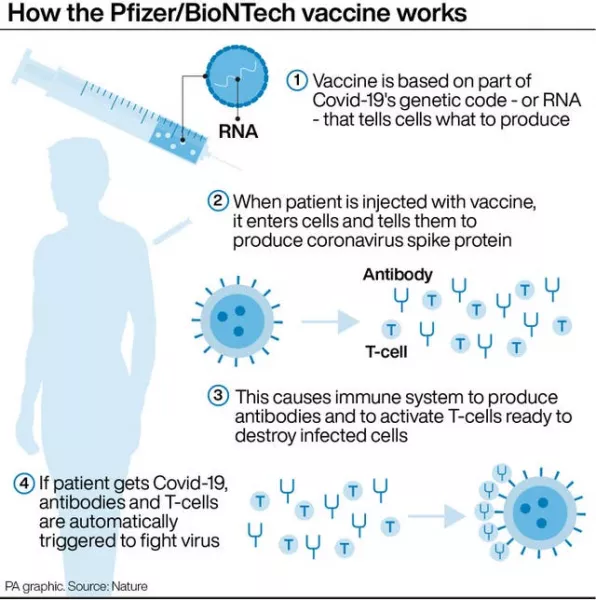
Originally, those having the vaccine were told their doses would be given 21 days apart but the government has now stretched the timeline for the second dose to between three and 12 weeks.
It did this following a recommendation from the Joint Committee on Vaccinations and Immunisation (JCVI) to give more people a first dose of the vaccine.
My legal-challenge to the government about delays to the second dose of Pfizer goes live today: see https://t.co/DI8eK9p7fL
Advertisement— Joan Bakewell (@JDBakewell) January 12, 2021
Ms Bakewell, 87, has instructed the law firm Leigh Day to start proceedings in response to the new dosing policy, and names the respondent as UK health secretary Matt Hancock.
The Leigh Day letter, marked “Urgent: Proposed claim for judicial review”, says: “Our client is fully supportive of the national effort to meet the exceptional challenges posed by the pandemic.
“Our client is, however, concerned that the government’s instruction to delay the provision of the second dose of the Pfizer vaccine is potentially unlawful and unsafe and would therefore impede rather than advance the pandemic response.”
The letter sets out three potential grounds for judicial review, including breach of the conditions of authorisation.
It says the move to delay doses appears to be “contrary to the instructions for use” that had been agreed between the UK regulator the Medicines and Healthcare products Regulatory Agency (MHRA) and Pfizer.
A second potential breach is that it “does not appear there was a proper or lawful basis for the Government to depart” from the MHRA’s assessment of the vaccine.
A third issue is a “breach of legitimate expectations”, with patients consenting to a course of medical treatment on the understanding they would get a second dose after 21 days.
Ms Bakewell, who received her first dose of the Pfizer vaccine in December, said: “Older people are in limbo: they need to know whether delaying the Pfizer vaccine is both safe and legal.
“I am bringing this case because I believe the government needs to make this clear.”
The move follows disquiet among some scientists about the 12-week delay, while Pfizer recommends the second dose of its jab is given after 21 days – as set out in its clinical trials.
Pfizer has said “the safety and efficacy of the vaccine has not been evaluated on different dosing schedules…there is no data to demonstrate that protection after the first dose is sustained after 21 days.”
Meanwhile, the World Health Organisation (WHO) said “there is very little empiric data from the trials that underpin this type of recommendation”.
Other scientists, writing in the British Medical Journal (BMJ), said the new policy is “not based on data from the trial, but on an assumption of what would have happened if the second dose hadn’t been given at 21 days”.
However, other experts have defended the policy, saying it will save the most lives from Covid-19.
Defended
England’s deputy chief medical officer, Professor Jonathan Van-Tam, said the data showed that patients got “almost complete protection” from their first dose of the Pfizer vaccine and could wait for their second dose.
He said JCVI analysis showed the vaccine was 89 per cent effective against Covid-19 in the period of 15 to 21 days after the first dose, and it was unlikely that such protection would decline severely in the 12 weeks after the jab.
“Simply put, every time we vaccinate someone a second time, we are not vaccinating someone else for the first time,” he wrote in the Mail on Sunday.

Stephanie Hill, solicitor at Leigh Day, said: “The Covid-19 pandemic has created exceptional challenges. Our client, like everybody, hopes that the vaccination programme will be rolled out as quickly as possible.
“It is crucial that any changes to the vaccination programme are made lawfully, in accordance with regulatory approval, and with the informed consent of patients.”
Ms Bakewell is fundraising for the legal costs for the case.







