The European Union has taken on vaccine producer AstraZeneca in a Brussels court, accusing the drugmaker of acting in bad faith by providing jabs to other nations when it had promised them for urgent delivery to the EU’s 27 member countries.
During an emergency hearing, the EU asked for the delivery of missing doses and accused AstraZeneca of postponing deliveries so the Anglo-Swedish company could service Britain, among others.
AstraZeneca denies any wrongdoing and said it has always done its best to fulfil delivery commitments.
Its contract with the European Commission foresaw an initial 300 million doses being distributed, with an option for another 100 million. The doses were expected to be delivered throughout 2021, but only 30 million were sent during the first quarter.
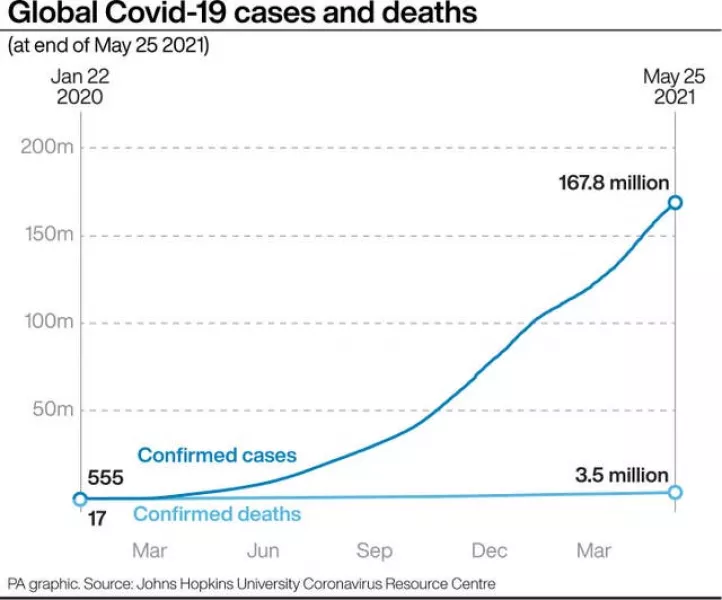
Deliveries have increased slightly since then but the commission says the company is set to supply 70 million doses in the second quarter when it had promised 180 million.
A lawyer for AstraZeneca said on Wednesday that “more or less 60 million doses” from the total order have been delivered so far.
EU lawyer Rafael Jafferali told the court that AstraZeneca expects to deliver the total number of contracted doses by the end of December, but “with a six-month delay, it’s obviously a failure”.
He asked the court to fine the drugmaker 10 million euros (£8.6 million) per infraction and to force AstraZeneca to pay 10 euros per dose for each day of delay as compensation for breaching the EU contract.
The EU has insisted its complaints are about deliveries only and it has no problems with the safety or quality of the vaccine. The jabs have been approved by the European Medicines Agency, the EU’s drug regulator.
The EU’s main argument is that AstraZeneca should have used production sites located within the bloc and in the UK for EU supplies as part of a “best reasonable effort” clause in the contract.
Mr Jafferali said the European Commission agreed to pay 870 million euros (£750 million) for the jabs and 50 million doses that should have been delivered to the EU went to third countries instead, “in violation” of the contract.
Charles-Edouard Lambert, another lawyer on the EU team, said AstraZeneca decided to reserve production at its Oxford site for Britain.
“This is utterly serious. AstraZeneca did not use all the means at its disposal. There is a double standard in the way it treats the UK and member states,” he said.
AstraZeneca said it informed the commission in a detailed production plan that the UK manufacturing chain would firstly be dedicated to British supplies. The company noted that delays in deliveries not only affected the EU but the whole world.
A lawyer representing AstraZeneca, Hakim Boularbah, said the company’s May 2020 agreement with the UK Government and Oxford University, the vaccine’s co-developer, to supply 100 million doses of vaccine at cost clearly gave priority to Britain.
“It’s very shocking to be accused of fraud,” he said, calling it “a groundless accusation”.
The EU also accused AstraZeneca of misleading the European Commission by providing data on the delivery delays that lacked clarity.
The company says it has fully complied with the agreement, arguing that vaccines are difficult to manufacture, with dozens of components produced in different nations, and it made its best effort to deliver on time.
“Unfortunately, to this date, more or less 60 million doses from the order have been delivered,” Mr Boularbah said, adding that AstraZeneca does everything it can to increase production and will deliver the 300 million of doses agreed to as soon as possible.
He played down the urgency claimed by the EU, saying 13 million AstraZeneca doses were stocked in EU member states. However, since the AstraZeneca vaccination takes two jabs up to 12 weeks apart, member states can reserve some supplies to make sure recipients get their second dose on time.
As part of an advanced purchase agreement with vaccine companies, the EU said it invested 2.7 billion euros (£2.3 billion), including 336 million euros (£290 million) to finance the production of AstraZeneca’s vaccine at four factories.
The long-standing dispute drew media attention for weeks earlier this year amid a deadly surge of coronavirus infections in Europe, when delays in vaccine production and deliveries hampered the EU’s vaccination campaign.
Cheaper and easier to use than a rival jab from Pfizer-BioNTech, the AstraZeneca vaccine developed with Oxford University was a pillar of the EU’s vaccine rollout, but the EU’s partnership with the firm quickly deteriorated amid accusations it favoured its relationship with British authorities.
While the UK made quick progress in its vaccination campaign thanks to its AstraZeneca supplies, the EU faced embarrassing complaints and criticism for its slow start.
AstraZeneca’s lawyers asked the judge to properly assess whether citizens in the EU cannot get vaccinated with the company’s jabs because of the delivery delays before determining whether the case should be settled urgently.
In total, the European Commission has secured more than 2.5 billion of vaccine doses with various manufacturers, but is now shying away from placing more orders with AstraZeneca.
A judgment is to be delivered at a later date.







