German health officials have agreed to restrict the use of AstraZeneca’s coronavirus vaccine in people under the age of 60, amid fresh concern over unusual blood clots reported in a tiny number of those who received the jabs.
Health minister Jens Spahn and state officials agreed unanimously to only give the vaccine to people aged 60 or older, unless they belong to a high-risk category for serious illness from Covid-19 and have agreed to take the vaccine despite the small risk of a serious side-effect.
The same option will be available to anyone who gets the jab at their GP, which will start to become possible later this month.

Mr Spahn told reporters: “In sum it’s about weighing the risk of a side-effect that is statistically small, but needs to be taken seriously, and the risk of falling ill with (coronavirus).”
The move follows the recommendations of Germany’s independent vaccine expert panel and comes after the country’s medical regulator released new data showing a rise in reported cases of an unusual form of blood clot in the head – known as sinus vein thrombosis – in recent recipients of the AstraZeneca vaccine.
The news is a further blow to the vaccine, which is critical to Europe’s immunisation campaign and a linchpin in the global strategy to get shots to poorer countries.
It comes less than two weeks after the EU drug regulator said the vaccine does not increase the overall incidence of blood clots following a similar scare.
The European Medicines Agency said at the time that the benefits of vaccination outweigh the risks, but it could not rule out a link between the shot and some unusual kinds of clots, and recommended adding a warning about possible rare side-effects.

Among those who will likely be eligible for the jab soon is German chancellor Angela Merkel.
The 66-year-old said that Tuesday’s decision was important for ensuring public trust in the vaccine campaign and that she would accept the shot made by the UK-Swedish company.
“When it’s my turn I’ll allow myself to be vaccinated, including with AstraZeneca,” Mrs Merkel said.
Several German regions – including the capital Berlin and the country’s most populous state, North Rhine-Westphalia – had already suspended use of the jabs in younger people earlier on Tuesday after the country’s medical regulator said its tally of the rare blood clots reported by March 29 had increased to 31.
Some 2.7 million doses of AstraZeneca have been administered in Germany so far.
Nine of the people affected died, and all but two of the cases involved women, who were aged 20 to 63, the Paul Ehrlich Institute said.
In a statement ahead of the announcement, AstraZeneca said tens of millions of people worldwide have received its vaccines, and noted that the EU regulator and the World Health Organisation concluded that the benefits of the jab outweigh the risks.
We recently released data from our COVID-19 vaccine US Phase III trial. We maintain our commitment to supplying our vaccine broadly and equitably at no profit during the pandemic. pic.twitter.com/a0wLTtLaUc
— AstraZeneca (@AstraZeneca) March 25, 2021
The company said it would continue to work with German authorities to address any questions they might have, while also analysing its own records to understand whether the rare blood clots reported occur more commonly “than would be expected naturally in a population of millions of people”.
The suspensions come as Germany, along with other European countries, is scrambling to ramp up its vaccine programme, which lags far behind those in the UK and US.
By Monday, some 13.2 million people in the country had received at least one dose of vaccine, while nearly four million had received both jabs.
Use of the AstraZeneca vaccine was temporarily halted in several European countries earlier this month over concerns about the rare blood clots.
After a review by medical experts at the European Medicines Agency, most European Union countries, including Germany, resumed use of the vaccine on March 19.
On Monday, Canada suspended use of the AstraZeneca vaccine in people under 55, citing new concerning data from Europe.
It's really important that every country, every individual focusses on doing the things that can keep the transmissions down, while we scale up vaccines.
— @WHO's Chief Scientist @doctorsoumya on what you can do to help reduce spread of #COVID19 variants: https://t.co/Mex4LngGg4 pic.twitter.com/X0w4keNERw— United Nations in India (@UNinIndia) March 31, 2021
“There is substantial uncertainty about the benefit of providing AstraZeneca Covid-19 vaccines to adults under 55 given the potential risks,” said Dr Shelley Deeks, vice chair of Canada’s National Advisory Committee on Immunisation.
Dr Deeks said the updated recommendations come amid new data from Europe that suggests the risk of blood clots is now potentially as high as one in 100,000, much higher than the one-in-a-million risk cited before.
Germany’s decision is likely to affect appointments made by tens of thousands of teachers and people with pre-existing conditions who received invitations to get vaccinated in Berlin in recent days.
Appointments for the AstraZeneca shot were available sooner than for those made by Pfizer-BioNTech and Moderna due to higher demand for those vaccines.
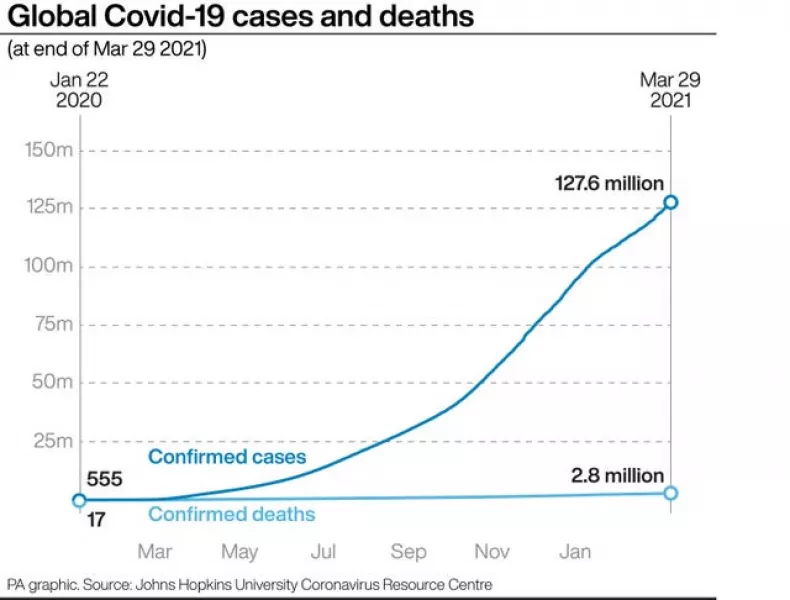
Scientists at the University of Greifswald, Germany, this week published the results of their investigation into the possible causes of the blood clots, saying the condition is similar to a side-effect seen in some patients who receive the blood thinning medication heparin.
The study, which has not been peer-reviewed yet, does not provide a conclusive explanation for why some people vaccinated with the AstraZeneca shot develop the rare blood clots.
Experts not involved in the study have said it offers important information to doctors.
Alice Assinger, a specialist in vascular diseases and blood clot research at the Medical University of Vienna, said there is a treatment for the rare clots.
However, others warned the German decision could harm the vaccine campaign.
“If people are not vaccinated, because use of the vaccine is suspended, or because of a drop in confidence in the vaccine caused by the decision, some of them will get ill from Covid-19; and some of them will die,” said Peter English, the former chairman of the British Medical Association’s public health medicine committee.
“Any decision to withhold the vaccine will directly cause excess, avoidable Covid-19 deaths.”