The Moderna Covid-19 vaccine has been given authorisation for use in the US by the country’s Food and Drug Administration (FDA).
The move marks the world’s first clearance for Moderna’s shots, and the vaccine is very similar to one from Pfizer and Germany’s BioNTech jab which is already being rolled out.
The two work “better than we almost dared to hope,” National Institutes of Health director Dr Francis Collins said, adding: “Science is working here, science has done something amazing.”
Early results of large, still unfinished studies show both the Pfizer/BioNTech and Moderna jabs appear safe and strongly protective although Moderna’s is easier to handle since it does not need to be stored at ultra-frozen temperatures.

The nation is scrambling to expand vaccinations as rapidly as Moderna and Pfizer can churn out doses. Moderna’s is for people 18 and older, Pfizer’s starts at age 16.
It’s just the beginning of “what we hope will be a big push to get this terrible virus behind us, although it will take many more months to get to all Americans,” Dr Collins said.
The FDA’s decision could help pave the way for other countries that are considering the Moderna vaccine.
European regulators could authorise its use as soon as January 6. Britain, Canada and a few other countries have already cleared the Pfizer-BioNTech shot, with a European Union decision due on Monday.
A second vaccine represents a ray of hope amid despair as the virus continues to spread unabated across the US even before holiday gatherings that are certain to further fuel the outbreak.
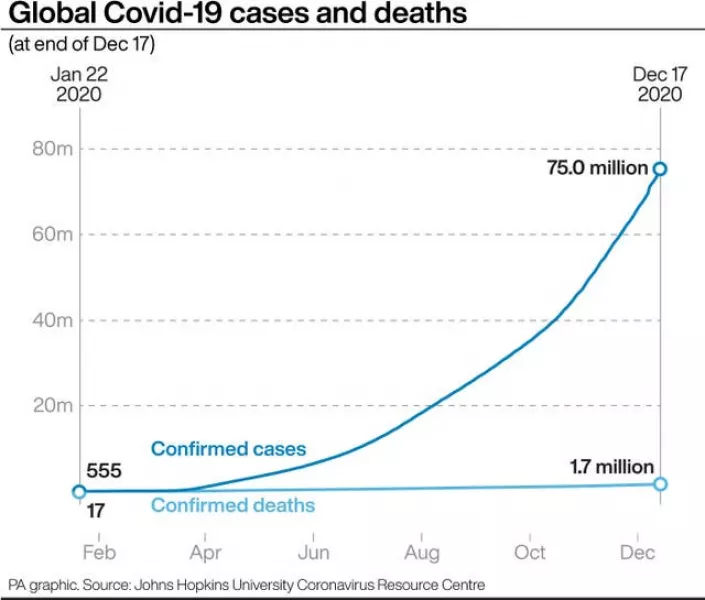
The pandemic has claimed more than 312,000 US lives and killed 1.7 million people worldwide.
New cases in the US are reaching around 216,000 per day on average, and deaths per day have hit all-time highs, surpassing 3,600 on Wednesday.







