Work is under way to tweak vaccines against the new concerning strain of Covid-19 that has sparked travel bans around the world.
The strain, named Omicron and designated a “variant of concern” by the World Health Organisation (WHO) on Friday, has reached Belgium after being discovered in South Africa.
The WHO warned that preliminary evidence suggests the variant has an increased risk of reinfection and may spread more rapidly than other strains.
A number of pharmaceutical firms have said they are now working to adapt their vaccines in light of the emergence of the variant.
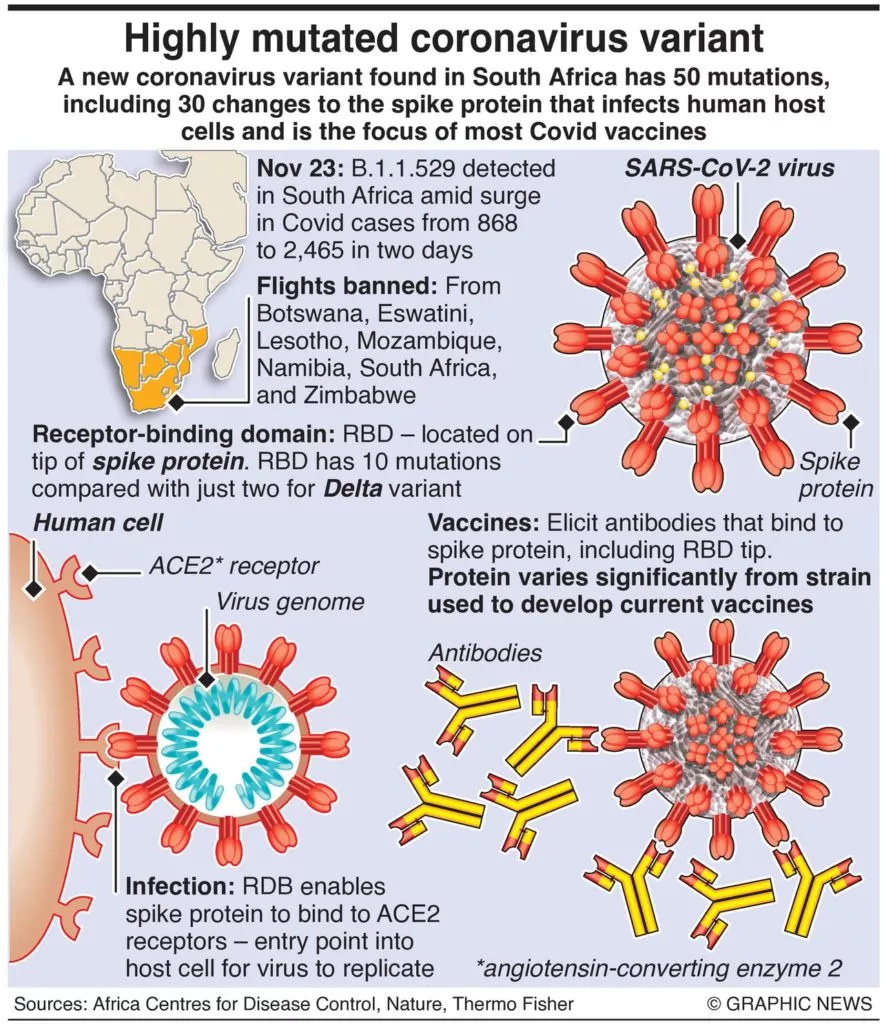
The EU, UK, US and Canada had all imposed travel restrictions on visitors from southern Africa ahead of the WHO adding the strain, also known as B.1.1.529, to its highest category for concerning variants on Friday evening.
WHO experts said there is early evidence to suggest Omicron has an “increased risk of reinfection” and its rapid spread in South Africa suggests it has a “growth advantage”.
Novavax said it has “already initiated development of a new recombinant spike protein based on the known genetic sequence of B.1.1.529 and will have it ready to begin testing and manufacturing within the next few weeks”.
Moderna said: “Since early 2021, Moderna has advanced a comprehensive strategy to anticipate new variants of concern.
“This strategy includes three levels of response should the currently authorised 50 µg (microgram) booster dose of mRNA-1273 prove insufficient to boost waning immunity against the Omicron variant.”
Pfizer and BioNTech said in the event of a variant which could escape the effects of the vaccines, the firm expects “to be able to develop and produce a tailor-made vaccine against that variant in approximately 100 days, subject to regulatory approval”.
AstraZeneca said it has “developed, in close collaboration with Oxford University, a vaccine platform that enables us to respond quickly to new variants that may emerge” and is “already conducting research in locations where the variant has been identified”.
The firm is also testing its antibody combination drug against the new variant and is “hopeful” it “will retain efficacy since it comprises two potent antibodies with different and complementary activities against the virus”.
No cases of the new strain have been detected in Ireland or the UK, however, the first case in Europe was confirmed on Friday in Belgium, heightening concerns on the continent. The strain has also previously been detected in Botswana, Hong Kong and Israel.
Marc Van Ranst, a virologist at the Rega Institute in Belgium, said a sample was confirmed as the variant in a traveller who returned from Egypt on November 11th before first showing symptoms 11 days later.
In response to the concerns, the Government approved travel restrictions on Friday evening, requiring Irish residents returning home from parts of southern Africa to undergo home quarantine and PCR testing.

The Government also announced the State is to align with the EU recommendation to apply the “emergency brake” and to discourage travel to or from Botswana, Eswatini, Lesotho, Mozambique, Namibia, South Africa and Zimbabwe.
The Department of Justice is updating visa requirements for those countries and the Department of Foreign Affairs has changed its travel advisory to “avoid non-essential travel” to these countries.
There are also plans to reintroduce the legislation for the mandatory hotel quarantine system (MHQ) with a view to re-establishing it in the coming weeks







