The head of the Food and Drug Administration (FDA) has said his agency will move to quickly authorise a second Covid-19 vaccine for the US to fight the pandemic, hours after the shot won the key endorsement of a government advisory panel.
FDA commissioner Stephen Hahn said in a statement that regulators have communicated their plans to pharmaceutical company Moderna, which co-developed the vaccine with the National Institutes of Health.
The announcement came after a panel of FDA advisers, in a 20-0 vote, ruled that the benefits of the vaccine outweighed the risks for those 18-years-old and up.
Once FDA’s emergency use authorisation is granted, Moderna will begin shipping millions of doses, earmarked for health workers and nursing home residents, to boost the largest vaccination effort in US history.
The campaign kicked off earlier this week with the first vaccine approved in the US, developed by Pfizer and BioNTech.
Moderna’s shot showed similarly strong effectiveness, providing 94% protection against Covid-19 in the company’s ongoing study of 30,000 people.
After eight hours of discussion over technical details of the company’s study and follow-up plans, nearly all panelists backed making the vaccine available to help fight the pandemic. One panel member abstained.
“The evidence that has been studied in great detail on this vaccine highly outweighs any of the issues we’ve seen,” said doctor Hayley Gans of Stanford University Medical Centre.
A second vaccine is urgently needed as coronavirus infections, hospitalisations and deaths climb to new highs ahead of the holidays.
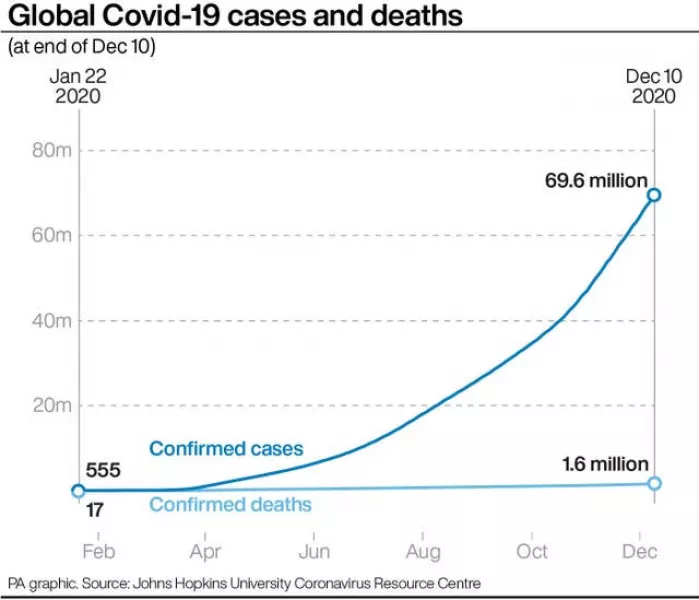
The US leads the world in virus totals, with more than 1.6 million confirmed cases and more than 310,000 reported deaths.
Moderna’s vaccine uses the same groundbreaking technology as Pfizer-BioNTech’s shot.
Most traditional vaccines use dead or weakened virus, but both of the new vaccines use snippets of Covid-19’s genetic code to train the immune system to detect and fight the virus. Both require two doses; Moderna’s is four weeks apart.







