The British government is putting £33.6 million (€36.8 million) towards the research, with trials set to begin in early January 2021.
At present, there are more than 240 coronavirus vaccine candidates being tested around the world.
Here is everything you need to know about the race to get a Covid-19 vaccine.
Of the 240 vaccines in early development, 44 are currently in clinical trials.
There are nine vaccine candidates in the phase three stage of clinical evaluation, in which doses are being given to thousands of people to confirm safety and effectiveness.
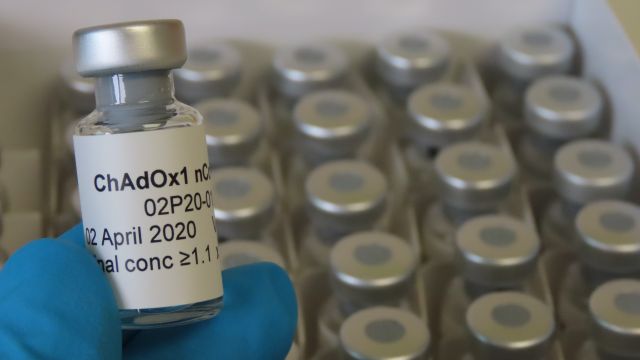
One such candidate is the Oxford vaccine, called ChAdOx1 nCoV-19, which uses a weakened version of a common cold virus (adenovirus) which causes infections in chimpanzees.
Other potential vaccines in phase three trials include ones from US drugs firm Moderna and US biotech company Novavax.
Meanwhile, China has also said four of its candidates are in phase three clinical trials.
However, no-one knows how effective any of these vaccines will be.
#COVID19 vaccine update 💉
Our vaccine trials have moved to the next phase 🙌
"The early clinical trials are progressing well and I would like to thank all those who are supporting this work, particularly our trial volunteers.” Find out more 👇https://t.co/xBAscJCYSw— Imperial College London (@imperialcollege) July 17, 2020
Advertisement
Aside from the Oxford vaccine, a coronavirus jab is also being developed by Imperial College London.
The Imperial vaccine is in phase one of clinical testing, where doses are being given to a small group of people to determine whether it is safe and to learn more about the immune response it provokes.
Pharmaceutical companies Sanofi and GlaxoSmithKline have also teamed up with the hope of making a Covid-19 vaccine available by the middle of next year.
The Sanofi/GSK candidate is currently in the phase two stage, where the vaccine is being given to hundreds of people so scientists can learn more about its safety and correct dosage.
Meanwhile, on Tuesday it was announced that volunteers in the UK could be exposed to the new coronavirus in controlled settings in a bid to speed up vaccine development.
Human challenge trials are expected to start in January 2021, pending approval from regulatory bodies and ethics committees.
NEW: We've just secured early access to 90m doses of two promising Covid-19 vaccine candidates from @Novavax and @JanssenGlobal
The UK has now secured six different vaccines - ensuring we have the best chance possible of finding one that works
👇 👇 👇https://t.co/x4eR9mL0iH— Alok Sharma (@AlokSharma_RDG) August 14, 2020
The European Commission has agreed to purchase of 300 million doses of the AstraZeneca vaccine, with an option to purchase 100 million more, on behalf of EU countries.
In August, the British government announced that the UK has secured access to six different Covid-19 vaccine candidates in development, representing some 340 million doses.
These potential vaccines include the Oxford, Novavax and GSK/Sanofi candidates, as well as ones being developed by Janssen (a pharmaceutical business owned by Johnson and Johnson) and French biotech company Valneva.
The UK has also secured 30 million doses of a vaccine that is being developed by German biotech firm BioNtech and US pharmaceutical company Pfizer.
The deals cover four different types of vaccines – adenoviral vaccines, mRNA vaccines, inactivated whole virus vaccines and protein adjuvant vaccines.
Adenoviral vaccines are weakened versions of adenoviruses while mRNA candidates are made up of small or inactivated doses of the whole disease-causing organism.
Inactivated whole virus vaccines, on the other hand, contain whole bacteria or viruses which have been killed, while protein adjuvant jabs are those where an adjuvant is added to some vaccines to enhance the immune response.
Should any of these candidates be approved, priority groups such as frontline health and care home workers, those with serious diseases, the elderly and ethnic minorities are first in line to receive a jab.
We are honored to collaborate with the #UK Government to advance #NVXCoV2373 into Phase 3 testing and provide 60 million doses for the UK. Read more here: https://t.co/XrZYcEC1QP. #COVID19 #vaccine #Novavax2020 pic.twitter.com/dAcL7Rapyk
— Novavax (@Novavax) August 14, 2020
A vaccine usually takes years, often decades, to develop but scientists working on potential coronavirus jabs are hoping to achieve the same amount of work in a few months.
Most experts are optimistic that a vaccine is likely to become available by mid-2021, which would be around 12-18 months after the the new coronavirus first emerged.
Peter Openshaw, from Imperial College London, who is co-investigator of human challenge studies project, said: “Watching this enormously dynamic vaccine landscape that is unfolding before us, I think that it is likely that we will get some answers pretty soon [as to] which vaccines might be effective.
“It may be that there will be several vaccines that are effective, perhaps in different groups or in different ways.”